Introduction: Allogeneic hematopoietic cell transplant (alloHCT) in elderly patients is a challenge, as with increasing age, there is higher incidence of co-morbidities and pre-frail status. In our recent study on alloHCT in patients 60 years (y) or older, those who were 70y or more had worse outcome and high 2-y non-relapse mortality (NRM) of 53% (Al-Shaibani E et al. Ann Hematol, 2023, 102: 917-926). We therefore implemented a number of changes to improve outcomes in elderly patients undergoing alloHCT. In this study, we analyze transplant outcomes in patients ≥ 70 y, and compare the earlier cohort with the more recent cohort.
Methods: This is a retrospective analysis of all consecutive patients 70y or older, who had an alloHCT from Jan 2015 to Dec 2022. We compared those who underwent an alloHCT from 2015-2019 (Cohort A, n=44) with those who had a transplant from 2020-2022 (Cohort B, n=40).
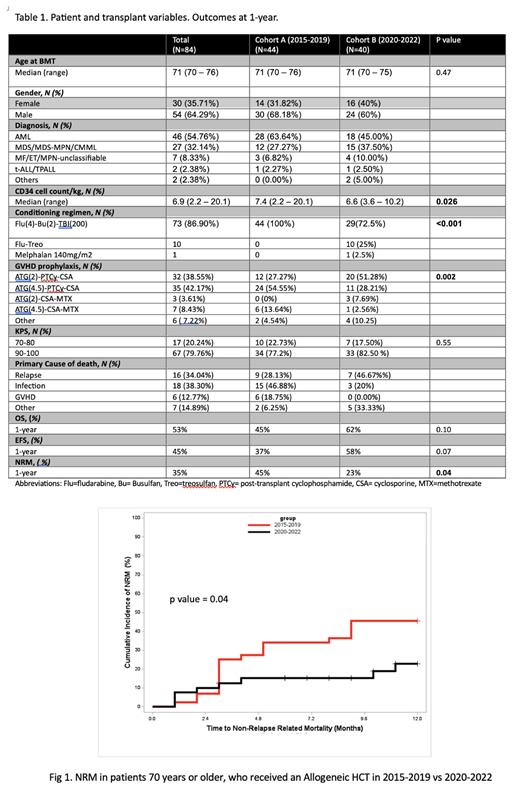
The median age of the patients was 71y (range 70-76). The underlying diagnosis was mainly a myeloid malignancy (AML, MDS, MPN, CMML, n=82; lymphoid (ALL, TPLL, n=2). There were 54 males. Stages of disease at transplant were in CR1 (n=51), CR2 (n=4) or stable disease (n=15, MDS, MPN or CMML pts). All patients received RIC regimens for conditioning including Flu4/Bu2/TBI200 (n=73), Flu4/Treo30 (n=7) and Flu/Treo42 (n=3). The GVHD prophylaxis regimens used included ATG2/PTCy/CSA (n=32), ATG4.5/PTCy/CSA (n=35), ATG4.5/CSA/MTX (n=7), ATG2/CSA/MTX (n=3). The donors were either matched related (n=10), matched unrelated (including minor mismatch) (n=57) or haploidentical (n=16). Graft source was peripheral blood in all except one who received bone marrow. (Details in Table).
The overall survival (OS), event free survival (EFS), NRM, cumulative incidence of relapse (CIR), incidence of acute and chronic GVHD, length of transplant hospitalization and the number of hospital re-admissions in first 6 months were assessed. The Kaplan Meier curves were plotted for OS and EFS for the overall sample, as well as stratified by the two eras (cohort A and B) and the differences assessed using log-rank tests. Cumulative incidence curves for NRM and CIR were generated, and differences assessed using Gray's K-sample.
Results: For all patients transplanted in 2015-2022, the 1-year OS was 53%, the 1-year EFS was 45% and the 1-year NRM was 35%. The median number of days of transplant hospitalization for the whole population was 33.5 days (range 16 - 116). The incidence of grade I-II aGVHD was 32%, and grade III-IV aGVHD was 9.5%; mild to moderate cGVHD was 14% and severe cGVHD was 2%. The 1-year CIR was approximately 18%.
Comparing the two cohorts A and B, the 1y OS was 45% vs 62% (p=0.10) respectively; the 1-year EFS was 37% vs 58% (p=0.07) respectively and the 1-year NRM was 45% vs 23% (p=0.04) respectively (Figures attached). The incidence of GVHD and CIR were similar in both cohorts, despite reducing the ATG dose in cohort B.
Conclusions: Our study shows that there were statistically significant improvements in NRM and a trend towards improvement in the OS and EFS in the patients transplanted during 2020-2022 compared to patients undergoing alloHCT from 2015-2019. This improvement may be due to one or more changes implemented in recent years, although it is not possible to identify a single factor. These changes were (a) introduction of Frailty testing in all patients (Salas MQ et al. BMT. 2021; 56:60-69). (b) capping the CD34 cell dose in grafts (c) introduction of Treosulfan for selected indications (MDS/CMML) (Pasic I et al. Leuk Res 2023, 128: 107131). (d) Letermovir for CMV prophylaxis (e) reducing ATG dose for GVHD prophylaxis and (f) focus on diet, nutrition, exercise. While alloHCT should be offered to medically eligible elderly patients, there is a special need to minimize the risks that are part of age related vulnerabilities.
Disclosures
Law:Actinium Pharmaceuticals: Research Funding. Kim:Novartis: Consultancy, Honoraria, Research Funding; BMS: Research Funding; Pfizer: Consultancy, Honoraria, Research Funding; Paladin: Consultancy, Honoraria, Research Funding; Merk: Consultancy; Sanofi: Consultancy, Honoraria. Lipton:ARIAD: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Mattsson:Takeda Canada Inc: Consultancy, Ended employment in the past 24 months, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria; Sanofi Canada: Honoraria, Other: advisory board; Magenta Therapeutics Inc: Consultancy, Honoraria; Medexus: Honoraria, Other: advisory board; Merck Canada Inc: Ended employment in the past 24 months, Honoraria, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal